Cyted’s diagnostic technology streamlines endoscopy services in secondary care
- Capsule sponge technology proven to alleviate resource pressure on delivering endoscopies by reducing demand in secondary care by 78% in the UK
- IQVIA calculated expected savings for the UK National Health Service of an estimated £421 per patient
An independent assessment by advanced analytics provider IQVIA in partnership with NHS England has found that Cyted’s minimally-invasive diagnostic technology reduced the demand for endoscopies in secondary care by 78%, improved the patient experience and decreased costs for the UK health system.
“The findings of this report illustrate the significant national impact of embedding our capsule sponge technology,” said Cyted CEO and Co-founder Marcel Gehrung. “It has allowed patients waiting for endoscopy to be effectively prioritised, enabling those in urgent need of diagnosis to be tested more quickly, reducing waiting lists and decreasing pressure on health systems. We look forward to expanding the adoption of this technology, helping detect disease earlier and improve patient outcomes.”
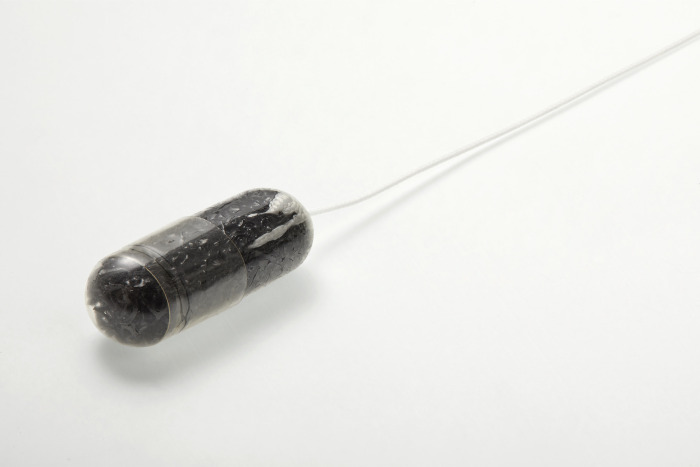
Cyted’s capsule sponge technology, EndoSign® is a non-endoscopic test used in the Heartburn Health Check. It sees patients swallow a capsule which collects cells from the oesophagus in a process that takes less than ten minutes. Analysis of these cells allows clinicians to monitor patients suffering from chronic heartburn and Barrett’s oesophagus - conditions that bring a higher risk of developing oesophageal cancer - and ensure that cancer is quickly detected and treated.
IQVIA’s report found that patient satisfaction levels were high, with 82% of patients agreeing that they were satisfied with their experience. Patients who had received an endoscopy before explained that the capsule sponge test was a preferable option to the current standard of care for diagnosing and monitoring patients with Barrett’s oesophagus. Wider deployment of the technology would serve to help improve patient experiences, alongside reducing waiting lists and easing pressures on patient pathways.
In reducing the number of endoscopies, this technology also reduced costs to the health system and alleviated the resource pressure in delivering endoscopy. The inclusion of capsule sponge technology in the diagnostic pathway resulted in a cost-saving of at least £421 per patient. Overall, according to IQVIA’s report, the introduction of the test at a national level would generate cost savings of up to £33 million over the course of five years for the NHS.
Swift diagnosis of Barrett’s oesophagus helps to identify more cancers earlier and faster. Oesophageal cancer is one of the most aggressive cancers, with over half a million people dying from the condition globally every year. By improving the efficiency and providing pre-screening capabilities, the capsule sponge technology of EndoSign® can help patients at risk of Oesophageal cancer. There are currently over 60 hospitals and 15 GP-based clinics offering EndoSign® testing across England, Scotland, and Wales.
Amanda Pritchard, NHS England’s chief executive, said: “Thousands of people have now benefited from this incredibly efficient test on the NHS. While the sponge on a string is small in size, it can make a big difference for patients.”
The project was undertaken by IQVIA over the course of the last two years in collaboration with National Health Service teams, Cyted, Medtronic, Cancer Alliances, Cambridge University and ENDOPREM.
Full press release from NHS England available here
A recording of the BSG webinar is available here