
Cyted receives 510(k) FDA clearance for EndoSign® technology
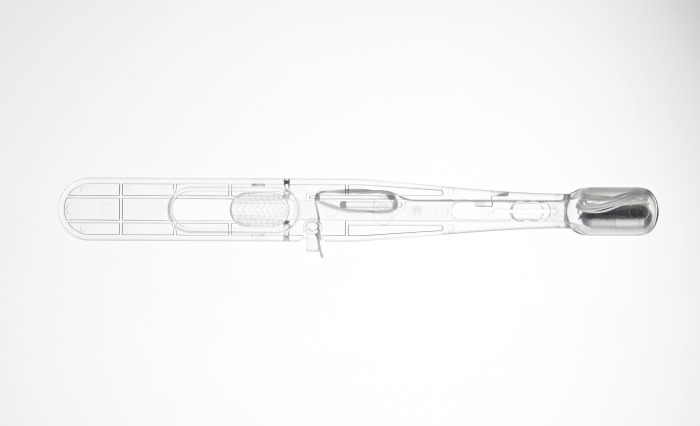
Health company Cyted announces that the company has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its EndoSign® cell collection device, which is a non-endoscopic capsule sponge device used to collect pan-esophageal samples. These samples are then sent for laboratory testing to detect esophageal pre-cancer and other conditions.
Marcel Gehrung, CEO and Co-founder of Cyted, expresses the importance of the milestone, stating “This clearance opens up significant opportunities for Cyted across new geographies and health systems and confirms our device is safe and effective for use in the US. Combined with novel biomarkers, Cyted’s potential to transform the way patients with chronic reflux are identified and monitored is significant and this clearance is a major step for our expansion.”
Effective immediately, this 510(k) FDA clearance is an exciting step to bringing the Heartburn Health Check with EndoSign®, into new markets. Commercialization plans are already underway facilitated by a new partnership with Devyser Genomic Laboratories announced earlier this month.
Cyted’s Chair and non-executive director Gail Marcus said “Building on their achievements in the United Kingdom, Cyted’s FDA clearance marks an exciting milestone for the global business. We are excited to continue to empower patients by providing innovative solutions for diagnosing gastrointestinal diseases.”
Gail Marcus, appointed as Chair of the Board in November, will be supporting Cyted’s expansion into the US. The EndoSign® technology is already widely used across all regions in the United Kingdom as part of the Heartburn Health Check which investigates patients with symptoms such as persistent heartburn and reflux to identify those at risk of developing esophageal cancer. Gail’s deep expertise in the US healthcare system will be vital to realizing Cyted’s potential in the US.
“The current paradigm of endoscopy-based screening to prevent esophageal cancer has not decreased the incidence of this deadly disease. Non-endoscopic screening offers a promising, cost-effective new direction to decrease cancer deaths. I am pleased that new modalities such as EndoSign® will be available for our patients.” said Nicholas J. Shaheen MD, MPH, Professor of Medicine and Chief of the Division of Gastroenterology and Hepatology at the University of North Carolina School of Medicine.
The EndoSign® technology has already demonstrated significant impact across multiple indications and applications in the digestive tract. Cyted has recently received a $1.3 million grant from the UK government to launch a precision medicine project for the diagnosis and treatment response prediction for the condition of Eosinophilic Esophagitis (EoE).
