News & Awards

.jpg)
Cyted Health today announces the appointment of Betsy Hanna as US President, where she will lead the company’s expansion into the US market. Betsy joins the company at a pivotal time, following FDA clearance for Cyted Health’s EndoSign® device earlier this year.
Betsy brings more than 20 years of healthcare executive leadership experience for diagnostics and medical device companies including genomic testing in oncology. She has a track record of leading strategy, marketing, and commercialization in global businesses and innovative start-up companies, successfully launching new products in the US and beyond. Betsy previously served as Agendia Precision Oncology’s Chief Commercial Officer and Executive Vice President. She received her MBA at Harvard Business School and holds a degree in Chemical Engineering from the University of Illinois.
“Betsy joins us to take on the significant work already underway to bring our products to the US market,” said Dr Marcel Gehrung, Cyted Health’s CEO and Co-Founder. “This is a crucial moment in what will be a core market for Cyted Health’s work, and Betsy will bring deep expertise and considerable experience to grow our presence in the US. She will be invaluable for guiding our team through successful commercialization and her appointment is another step to expanding our footprint globally.”
The FDA’s clearance of EndoSign® in February was one of a series of recent milestones achieved by Cyted Health in the US. It followed a partnership with Devyser Genomic Laboratories to conduct the lab analyses announced in January. In November last year, Gail Marcus was appointed Chair of the Board, bringing her vast knowledge of the US healthcare system and supporting Betsy to deliver this year’s strategic plan.
Cyted Health is a gastrointestinal health company that has developed EndoSign®, a minimally invasive test to detect and monitor for signs of pre-cancer and other diseases. Testing can be completed in minutes in a nurse-led clinic. EndoSign® is already widely used across the UK to identify Barrett’s esophagus – a precursor to esophageal cancer – and the risk of patients developing cancer in the future.
“I look forward to leading Cyted Health’s US arm and contributing to the fight against esophageal cancer,” said Betsy Hanna. “EndoSign® can transform current methods for monitoring patients and make sure conditions are detected earlier and treated faster. I am highly confident that we can make a significant impact bringing this technology to US patients.”

Read the full case study here.
The national implementation of capsule sponge testing in Scotland has delivered significant improvements for the care of upper gastrointestinal patients. The testing programme launched in 2020 to improve the quality of care and diagnostic waiting times for patients with Barrett’s oesophagus and those at risk of oesophageal cancer.
The challenge
The onset of the COVID-19 pandemic interrupted Scotland’s endoscopy services, resulting in insufficient capacity to deliver timely procedures for patients requiring surveillance for Barrett’s oesophagus. Delays to surveillance increase the risk of cancer progression being missed until the later stages. This prompted the search for non-endoscopic options with strong clinical evidence, with NHS Scotland opting to use the capsule sponge test.
The solution
During the pandemic NHS Scotland began the national-level implementation of capsule sponge testing, to find a way to fast-track patients with signs of cancer for treatment and schedule patients at medium-to-low risk for their next surveillance procedure appropriately. As of January 2024, over 6000 tests have been performed throughout Scotland, with the test acting as a triage tool to identify patients requiring further investigation with endoscopy.
Key findings
Capsule sponge testing has cut patient waiting times significantly for NHS Scotland, while enriching endoscopy lists and boosting dysplasia detection rates to help clinicians identify more at-risk patients. This has released endoscopy resources for other complex procedures, such as bowel cancer screening.
- Using the test for screening has helped Scotland reduce the demand for follow up endoscopies by 77%.
- A national evaluation predicted cost savings of £700k in the first year and £3.3m over 5 years.
- NHS Scotland reduced median waiting times for endoscopy from 9 months to 5 months.
- From under 10% to over 50% concerning pathologies found at endoscopy.
Conclusion
By delivering high-quality care with fewer endoscopies, NHS Scotland has demonstrated what state-of-the-art Barrett’s surveillance could look like, prompting clinical teams across the UK and Europe to consider how to make a difference in the earlier detection of oesophageal cancer.

- Cyted and Microsoft Research collaborated to build novel AI models for efficient screening of cancer cells that could reduce pathologist workload by as much as 63%
- Nature Communications published an article detailing the research
Cambridge based health company Cyted announced a publication in Nature Communications on research conducted in collaboration with Microsoft Research developing machine learning tools to enable earlier and faster detection of Barrett’s esophagus, a precursor to cancer.
“We have been delighted to collaborate with Microsoft Research to push the boundaries of what’s possible in medical imaging and screening technologies, creating optimal efficiencies from start to finish of the testing process” said Dr Marcel Gehrung, CEO and Co-founder at Cyted. “As we continue to expand, machine-learning will be an important tool to support our goals and make early cancer detection more accessible."
Esophageal cancer is one of the deadliest cancers with a five-year survival rate of less than 15%. Detecting Barrett’s esophagus, a precancerous condition, helps identify patients who are at a higher risk of developing esophageal cancer. Monitoring patients with this condition offers the best chance of early cancer detection and therefore earlier treatment of esophageal cancer, dramatically improving survival rates to 9 in 10 people surviving after 5 years.
The article titled “Enabling large-scale screening of Barrett’s esophagus using weakly supervised deep learning in histopathology” is an example of the innovative research that Cyted and Microsoft Research are advancing to optimize clinical workflows and improve the capabilities of earlier identification of cancer with technology.
Cyted’s capsule sponge technology, EndoSign® is a non-endoscopic test that collects cells from the esophagus in a process that takes less than ten minutes. Analysis of these cells is conducted by pathologists to identify biomarkers - a measurable biological indicator of the likelihood of a cancer developing.
Artificial Intelligence is being used to optimize the screening process by leveraging machine learning to help triage the large sets of pathology images. This saves precious pathologist time by screening out negative cases and controlling the quality of digitalized samples, cutting the pathologist’s workload down by as much as 63%.
“Microsoft Research seeks to advance science and technology to benefit humanity, often by identifying new applications for leveraging AI and machine learning,” said Javier Alvarez-Valle, Senior Director of Biomedical Imaging at Microsoft Research. “Collaborating with Cyted and sharing our work via open source will empower researchers and clinicians around the world to leverage this technology in their fight against cancer.”
The full article in Nature Communications can be viewed here.
A blog post written by Microsoft Research can also be found here.
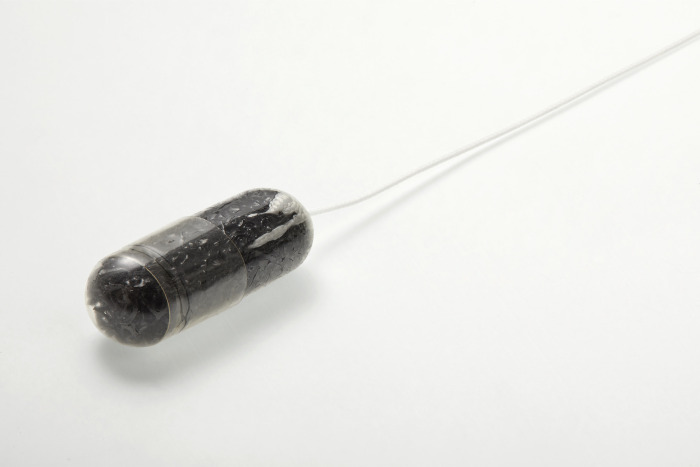
- Capsule sponge technology proven to alleviate resource pressure on delivering endoscopies by reducing demand in secondary care by 78% in the UK
- IQVIA calculated expected savings for the UK National Health Service of an estimated £421 per patient
An independent assessment by advanced analytics provider IQVIA in partnership with NHS England has found that Cyted’s minimally-invasive diagnostic technology reduced the demand for endoscopies in secondary care by 78%, improved the patient experience and decreased costs for the UK health system.
“The findings of this report illustrate the significant national impact of embedding our capsule sponge technology,” said Cyted CEO and Co-founder Marcel Gehrung. “It has allowed patients waiting for endoscopy to be effectively prioritised, enabling those in urgent need of diagnosis to be tested more quickly, reducing waiting lists and decreasing pressure on health systems. We look forward to expanding the adoption of this technology, helping detect disease earlier and improve patient outcomes.”
Cyted’s capsule sponge technology, EndoSign® is a non-endoscopic test used in the Heartburn Health Check. It sees patients swallow a capsule which collects cells from the oesophagus in a process that takes less than ten minutes. Analysis of these cells allows clinicians to monitor patients suffering from chronic heartburn and Barrett’s oesophagus - conditions that bring a higher risk of developing oesophageal cancer - and ensure that cancer is quickly detected and treated.
IQVIA’s report found that patient satisfaction levels were high, with 82% of patients agreeing that they were satisfied with their experience. Patients who had received an endoscopy before explained that the capsule sponge test was a preferable option to the current standard of care for diagnosing and monitoring patients with Barrett’s oesophagus. Wider deployment of the technology would serve to help improve patient experiences, alongside reducing waiting lists and easing pressures on patient pathways.
In reducing the number of endoscopies, this technology also reduced costs to the health system and alleviated the resource pressure in delivering endoscopy. The inclusion of capsule sponge technology in the diagnostic pathway resulted in a cost-saving of at least £421 per patient. Overall, according to IQVIA’s report, the introduction of the test at a national level would generate cost savings of up to £33 million over the course of five years for the NHS.
Swift diagnosis of Barrett’s oesophagus helps to identify more cancers earlier and faster. Oesophageal cancer is one of the most aggressive cancers, with over half a million people dying from the condition globally every year. By improving the efficiency and providing pre-screening capabilities, the capsule sponge technology of EndoSign® can help patients at risk of Oesophageal cancer. There are currently over 60 hospitals and 15 GP-based clinics offering EndoSign® testing across England, Scotland, and Wales.
Amanda Pritchard, NHS England’s chief executive, said: “Thousands of people have now benefited from this incredibly efficient test on the NHS. While the sponge on a string is small in size, it can make a big difference for patients.”
The project was undertaken by IQVIA over the course of the last two years in collaboration with National Health Service teams, Cyted, Medtronic, Cancer Alliances, Cambridge University and ENDOPREM.
Full press release from NHS England available here
A recording of the BSG webinar is available here
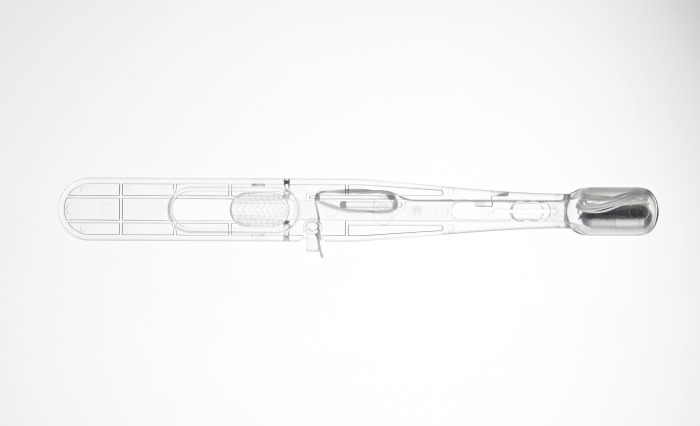
Health company Cyted announces that the company has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its EndoSign® cell collection device, which is a non-endoscopic capsule sponge device used to collect pan-esophageal samples. These samples are then sent for laboratory testing to detect esophageal pre-cancer and other conditions.
Marcel Gehrung, CEO and Co-founder of Cyted, expresses the importance of the milestone, stating “This clearance opens up significant opportunities for Cyted across new geographies and health systems and confirms our device is safe and effective for use in the US. Combined with novel biomarkers, Cyted’s potential to transform the way patients with chronic reflux are identified and monitored is significant and this clearance is a major step for our expansion.”
Effective immediately, this 510(k) FDA clearance is an exciting step to bringing the Heartburn Health Check with EndoSign®, into new markets. Commercialization plans are already underway facilitated by a new partnership with Devyser Genomic Laboratories announced earlier this month.
Cyted’s Chair and non-executive director Gail Marcus said “Building on their achievements in the United Kingdom, Cyted’s FDA clearance marks an exciting milestone for the global business. We are excited to continue to empower patients by providing innovative solutions for diagnosing gastrointestinal diseases.”
Gail Marcus, appointed as Chair of the Board in November, will be supporting Cyted’s expansion into the US. The EndoSign® technology is already widely used across all regions in the United Kingdom as part of the Heartburn Health Check which investigates patients with symptoms such as persistent heartburn and reflux to identify those at risk of developing esophageal cancer. Gail’s deep expertise in the US healthcare system will be vital to realizing Cyted’s potential in the US.
“The current paradigm of endoscopy-based screening to prevent esophageal cancer has not decreased the incidence of this deadly disease. Non-endoscopic screening offers a promising, cost-effective new direction to decrease cancer deaths. I am pleased that new modalities such as EndoSign® will be available for our patients.” said Nicholas J. Shaheen MD, MPH, Professor of Medicine and Chief of the Division of Gastroenterology and Hepatology at the University of North Carolina School of Medicine.
The EndoSign® technology has already demonstrated significant impact across multiple indications and applications in the digestive tract. Cyted has recently received a $1.3 million grant from the UK government to launch a precision medicine project for the diagnosis and treatment response prediction for the condition of Eosinophilic Esophagitis (EoE).

The BEST4 trial, designed to evaluate the potential of a national screening programme, started today. It seeks to explore if our Heartburn Health Check using the minimally invasive test, EndoSign, can prevent deaths from esophageal cancer when offered as a screening test to people on long-term medication for heartburn.
“This is another major step forward for our technology. The trial will evaluate the potential for a national screening programme and explore if our Heartburn Health Check using the minimally invasive test, EndoSign, can be used to prevent esophageal cancer when offered as a screening test to people on long-term medication for heartburn” said Cyted’s CEO and co-founder, Marcel Gehrung, “Our test is already deployed in over 70 hospitals and GP surgeries all across the UK and demonstrates how innovation can have a real and lasting impact on our health system, reducing waiting lists and ultimately saving lives.”
With Cyted’s non-invasive capsule sponge test continuing to prove its effectiveness at diagnosing Barret’s esophagus in the UK, the trial could pave the way for a test to be established as a routine screening programme to detect the condition, which can lead to esophageal cancer.
The trial builds on decades of research led by the Director at the Early Cancer Institute and Cyted’s co-founder, Professor Rebecca Fitzgerald. She and the team at Cyted invented and refined the capsule sponge test.

Tim Cowper, 49, a brewer from Cambridge, has had acid reflux, or heartburn, every night since he was 16. A routine health check while he was at university resulted in the shock diagnosis of Barrett’s esophagus. After his diagnosis, he has been monitored ever since. Tim said:
“I was alarmed when I was told that having Barrett’s meant having pre-cancerous cells in my gullet. Cancer is never a nice word to hear, especially when you are so young, but luckily, I’ve had my condition monitored.”
“Since my diagnosis, I’ve been going for an endoscopy at least once every three years to monitor my esophagus. It is not pleasant at all. Each time, I have a thick tube pushed down through my mouth, and I can feel every single one of the biopsies taken by the camera. Swallowing a capsule sponge is a much better experience, and I now get the test before my regular endoscopy appointment.”
The second stage of the trial, BEST4 Screening, opens in the summer and will recruit 120,000 people aged over 55 on long-term treatment for heartburn.
The multi-million-pound trial is jointly funded by Cancer Research UK and the National Institute for Health and Care Research.

The nominations recognise the entrepreneurial growth of the company, as well as the transformative impact the capsule sponge test and biomarker discovery process has had on a number of NHS sites.
From September to November, Cyted and our NHS partners were named as finalists in the following awards:
- Innovate Awards - Nominated for Outstanding Contribution to Population Health Through Innovation for the CYTOPRIME Project.
- https://www.innovatehealthcareawards.co.uk/finalists-2023
- LaingBuisson Awards - Nominated for Innovation In Health Tech.
- https://laingbuissonawards.com/the-2023-finalists/
- OBN Awards - Nominated for Best Established MedTech Company
- https://obn.glueup.com/event/obn-awards-2023-74448/2023-winners--finalists.htm
- Healthcare Honours Award - CYTOPRIME 1 Project nominated for the Outstanding innovation award alongside one NHS customer.
- https://www.healthcarehonours.com/shortlist-2023
- Barclaycard Entrepreneurship Award - Eagle Labs Innovation Award.
- https://www.barclays.co.uk/business-banking/sectors/entrepreneurs/awards/
- NHS Parliamentary Awards - One NHS customer named as finalists for The Excellence in Health Care Award for CYTOPRIME1.
- https://www.england.nhs.uk/nhs-parliamentary-awards/about/regional-winners
- HSJ Awards - Two NHS customers nominated for Modernising Diagnostics
- https://awards.hsj.co.uk/winners-2023
- HSJ Partnership Awards - One NHS customer named as finalists for Diagnostic Project of the Year for CYTOPRIME1.
- https://partnership.hsj.co.uk/finalists-2024

Four NHS sites have been named in the nominations, each using Cyted’s technology to tackle key challenges such as providing increased capacity, accessibility, and faster turnaround to improve care.
From ICB, system-wide initiatives and studies, to Trust and local level projects, the nominations demonstrate a wide range of implementations and the growing enthusiasm for capsule sponge testing.
Cyted’s CYTOPRIME1 project was recognised in a number of the nominations for both its scale and the success of the partnership between the company and the NHS sites, helping to transform endoscopy care pathways across Lancashire and South Cumbria.
The nominations add to the growing list of accolades for the company and support for capsule sponge testing seen in 2023. Cyted will continue building on this momentum in what promises to be another successful year of collaboration and innovation in 2024.


The gastrointestinal health company Cyted has received a £1 million grant from Innovate UK to launch its first major precision medicine project for diagnosis and treatment response prediction for the condition Eosinophilic Esophagitis (EoE).
The non-endoscopic EndoSign® platform is already widely used in the Heartburn Health Check to identify patients at risk of developing oesophageal cancer. This new programme is an important first step to increase the impact of non-endoscopic testing and make care for digestive diseases accessible to more patients.
"This grant allows us to build on our existing diagnostic technology to help many more people with undiagnosed or ongoing esophageal conditions,” said Marcel Gehrung, CEO & Co-Founder of Cyted. “Eosinophilic Esophagitis is treatable, but often undetected until a patient develops complications. Our EndoSign® platform will make a real difference to patients by decreasing the need for endoscopy and improving treatment decisions.”
EoE is a chronic inflammatory condition of the oesophagus and affects up to 1 in 1000 individuals and is responsible for a significant cost burden to healthcare systems. The condition can result in damage to the oesophagus, causing difficulty swallowing, pain, and even medical emergencies when food gets stuck. The developed test will also support clinicians in assessing a patient’s likelihood of responding to associated drug or dietary therapy, indicating the appropriate treatment for EoE patients. This will improve patient outcomes as well as reducing the time and cost associated with more invasive and expensive endoscopies.
The Innovate UK grant has been awarded as part of the Advancing Precision Medicine programme, aimed at driving UK productivity and economic growth through providing facilities and funding to test, demonstrate and evolve innovation in the health sector.
With the support of this grant, Cyted aims to use its expertise in non-endoscopic technologies and biomarker discovery to develop new tests to diagnose EoE in patients, and support treatment decisions in cases where the condition has already been diagnosed.
For more on the Innovate UK Grant, please clickhere
Recognizing Excellence: Our Awards and Accolades

