
Non-endoscopic diagnostics for GI care
We are a leading gastrointestinal health company transforming access to digestive health for everyone, everywhere.
Explore
Impact
Earlier matters
Upper GI diseases and cancers affect millions of people globally, every year. With our non-endoscopic diagnostic platform, we are transforming care for digestive diseases by ensuring earlier detection and improving access.
1 in 10
people suffer from upper GI diseases

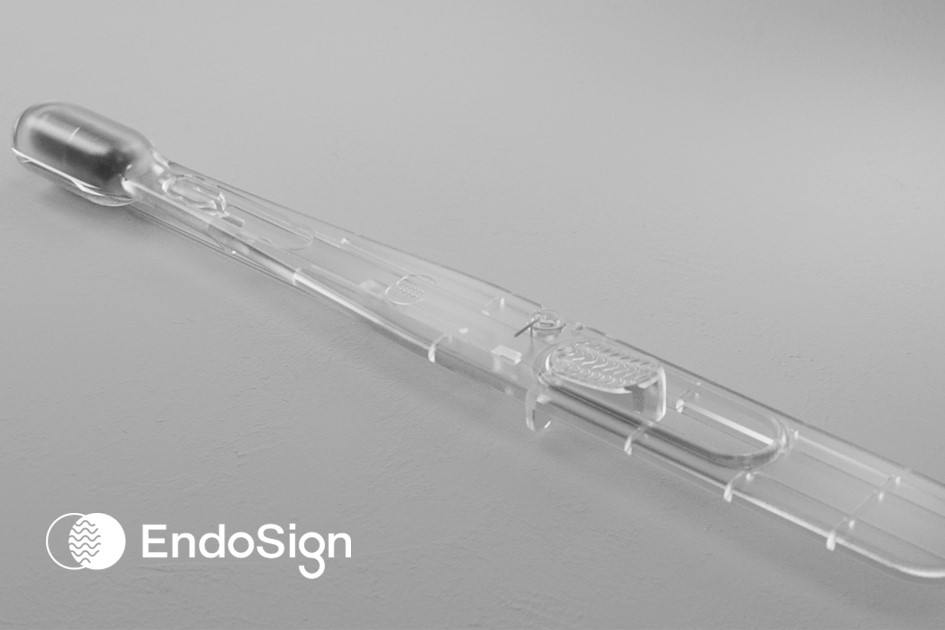
EndoSign® is a fast and simple test to protect esophageal health by detecting and monitoring for signs of pre-cancer and other diseases.
Find out more:
Recent news
View All
In partnership with
Cyted is proud to collaborate with a network of esteemed partners and investors who share our vision for innovative healthcare solutions.











Get in touch for more information about our early cancer test and data-driven diagnostics.
Get in touch



.jpg)

